For CROs and clinical sites
Clinical trial software for CROs and clinical sites
You know it all too well. In multicenter clinical trials, swaths of unstructured, sensitive data can quickly cause you to lose sight of the bigger picture: delivering life-changing therapies to patients.
That’s why we made the RAYLYTIC Platform – an all-in-one platform for the automated, centralized, and compliant collection and management of clinical study data.
Improve the quality of your data - fully automatically
Individually powerful. Together: Transformative. The RAYLYTIC Platform contains flexible modules for automatic data capture and AI-based image analysis.
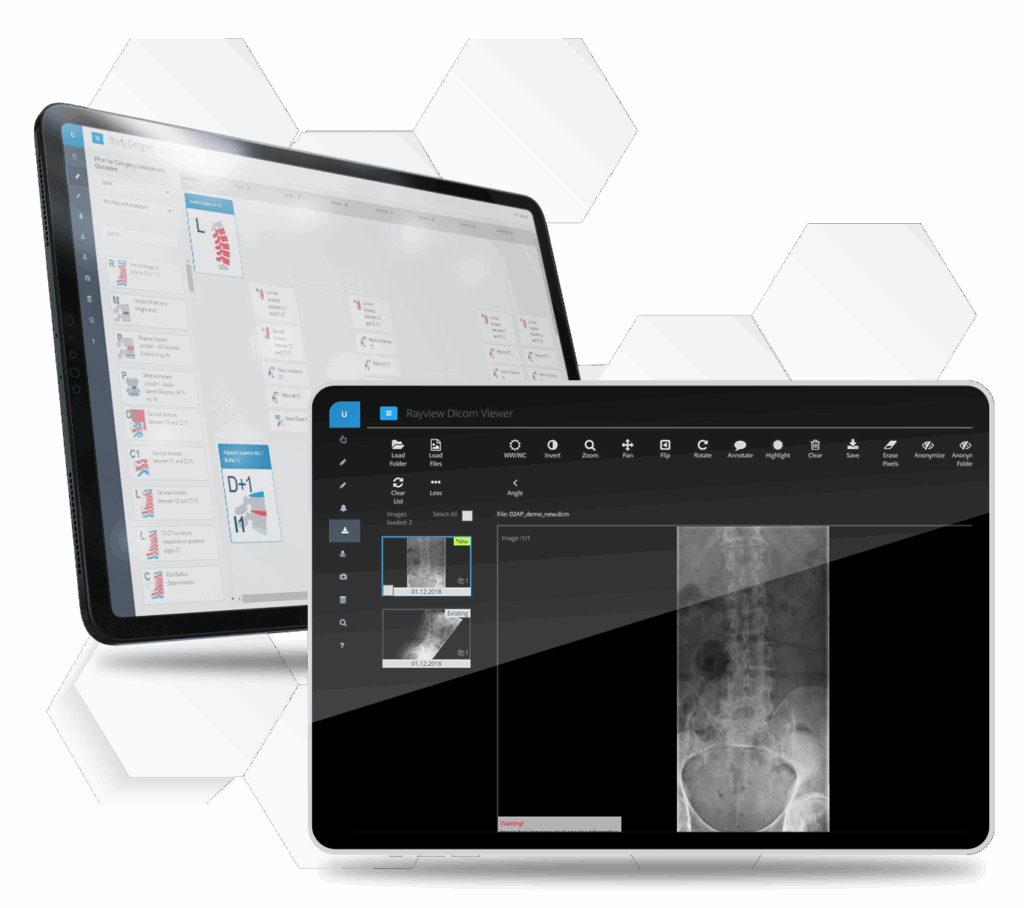
Compliantly upload and transfer medical imaging data.
- Integrated DICOM viewer
- Integrated quality controls
- Automatic image anonymization
Imaging core lab services
If needed, RAYLYTIC’s team of experts can support with:
- Consultations for study design, endpoint selection, and data interpretation
- Project management and on-call support
- A network of experienced radiologists
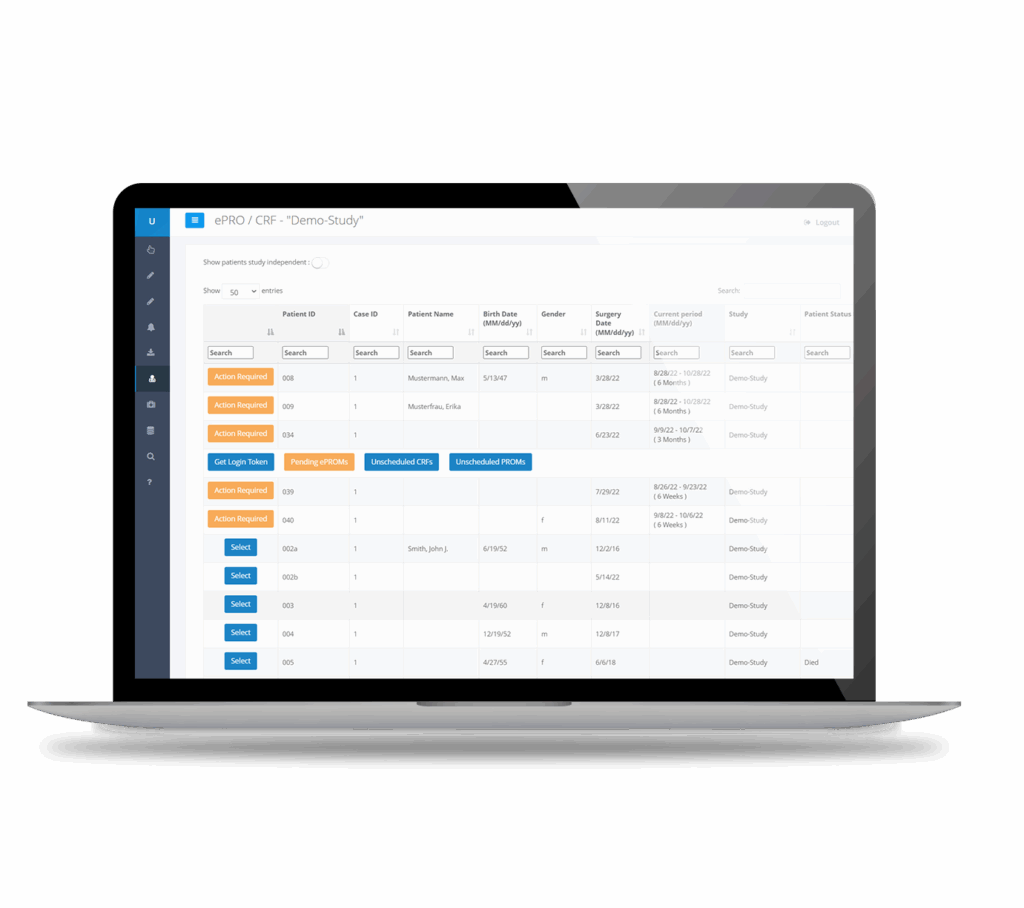
Electronic data capture
(EDC)
Collect complete and consistent ePRO, eCRF, and imaging data sets
- ePROs for all medical specialties
- Automated data input validation
- Customizable eCRFs and adverse event forms
Reduce manual tasks with an advanced degree of process automation.
- Automated notifications to patients and sites
- Real-time status of study results and data completeness
- Integratable with EHRs and scheduling software
Finally, a clinical trial management software that can do both.
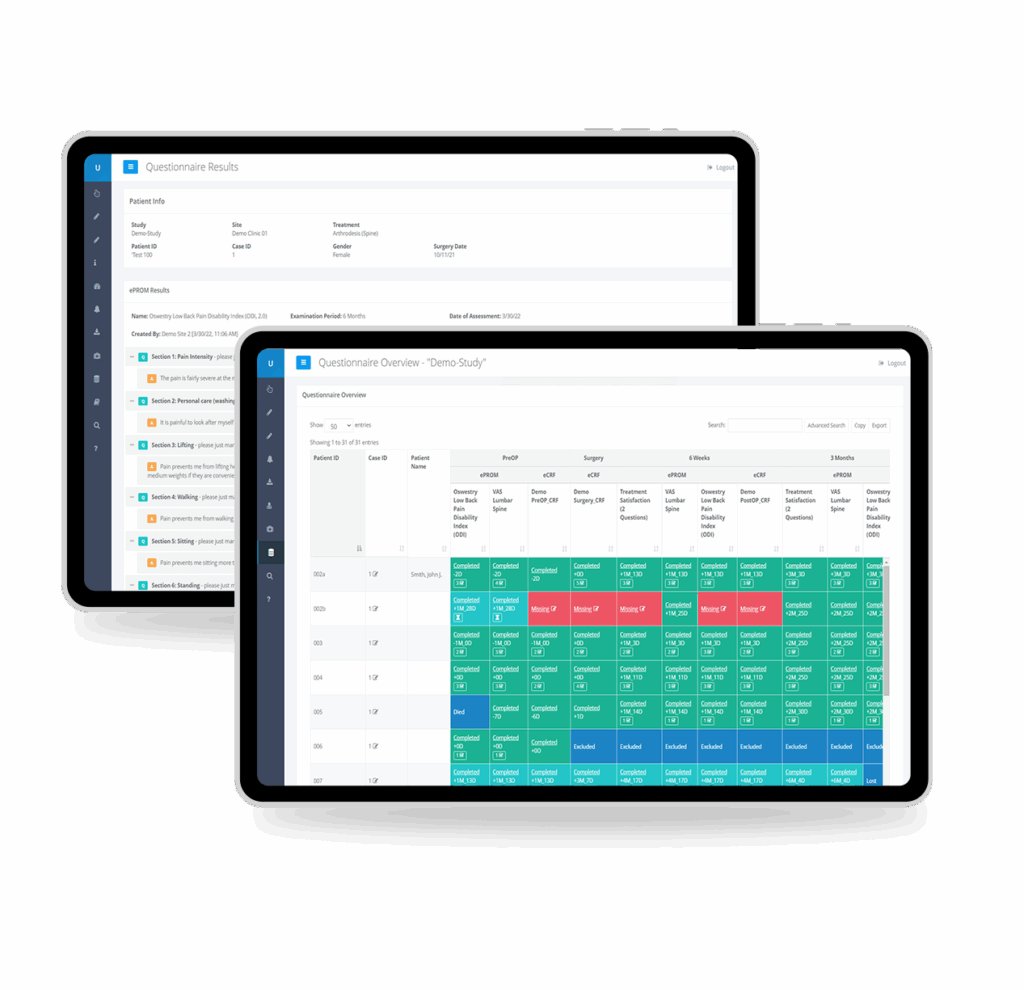
Measure patient-reported, clinical, and radiographic outcomes in a single, easy-to-use platform.
AI-supported medical image analysis
Our platform combines automated data collection with AI-based image analysis to provide you with consistent and accurate data for your regulatory submissions.
Automated and precise evaluation of over 150 radiographic parameters, including Range of Motion (RoM), implant wear and migration, and translational AP-motion
The UNITY platform checks the correctness of uploaded imaging data to ensure 1:1 adherence to study protocols.
Automated browser-based de-identification of imaging data ensures your study remains compliant with stringent regulatory and patient data protection requirements.
360° insights for sponsors and sites alike
Using our clinical trial software, CROs and clinical sites can run projects efficiently by reducing study build time, data inconsistencies, and complications during mid-study changes.
From protocol definition to data capture, analysis, statistical reporting, and close-out, our integrated “single-source-of-truth” approach means you benefit from full transparency and compliance to study protocols.
Uncomplicated dashboards and customizable reports help you deliver sponsors and sites data into enrollment and retention figures, adverse events, and data status.
UNITY facilitates straightforward communication between sites, patients, and sponsors with automated notifications – ensuring the right data is collected at the right time.
Clinical trial management software designed with sites in mind
Our clinical trial management software enables sites to gather data, enhance their workflows, and work with fewer hurdles.
Sponsors can easily trigger and manage queries. Communicating with site users is as easy as sending a text message.
Identify correlations between symptoms and outcomes faster: the UNITY platform enables users to automatically assign medical codes. Auto-coding accounts for synonyms and detects coding conflicts.
Interface between system landscape(s) (HIS, LIS, RIS, PACS) and UNITY based on HL7 v2, HL7 v3, CDA and FHIR. UNITY is available as a white label product, so users can navigate the software like they do their inhouse systems.
Better data. No sacrifices.
See how our partners are using the RAYLYTIC Platform to achieve success across diverse regulatory pathways.
Find the right operating mode for your trial
To suit the demands of your clinical trial best, the platform is available as a fully web-based or clinic-integrated version.
Fully web-based
The web-based, software as service (SaaS) option allows sites to start collecting clinical trial in just a matter of minutes.
Our onboarding process is short and uncomplicated. After a brief online webinar, site users can begin uploading DICOMs and collecting ePRO and eCRF data.
The ePRO module is a class I medical product in Europe according to the European Medical Device Regulation 2017/745/EU, and the EDC module is FDA 21 CRF Part 11 compliant in the United States.
Clinic integration on demand
Designed for large clinical sites, the clinic-integrated version merges seamlessly with electronic health records, hospital information systems, scheduling software, and archiving systems via our CLIO integration server.
Patient data always stay where they belong: the hospital. Our hybrid integration architecture ensures that only highly encrypted information is transmitted and cannot be traced back to patients.
Your successful clinical trial. Our know-how.
Rapid onboarding
Better patient engagement
Easy operation
Reclaimed time
First-rate support
Medical imaging data, like X-rays, MRIs, and CT scans, produce direct insights into the efficacy of medical devices and implants, helping to accelerate clinical trials. However, additional study-related and regulatory complexity often accompany clinical trial management in studies including medical imaging data.
Our scalable, modular, and cloud-based platform helps CROs save time and costs in studies with imaging endpoints and stringent regulatory considerations. Thanks to browser-based image de-identification, sites can compliantly upload, transfer, store, and analyze structured imaging data, like DICOM files. CROs, on the other hand, can rely on the platform’s integrated AI-based imaging classification to automatically improve data quality. This lowers the time and effort needed for efficient clinical trial management.
Furthermore, we our management and data storage processes are certified according to ISO 27001 and ISO 13485