For medical device trial sponsors
Next-Generation Clinical Trial Software for Medical Device Studies
Our platform integrates AI-driven imaging analysis, real-time data validation, and unified EDC and PROMs management to enable medical device manufacturers to accelerate regulatory submissions by ensuring rigorous protocol adherence, data integrity, and comprehensive patient outcome tracking.
Fast-track your device approvals and cement your scientific legacy.
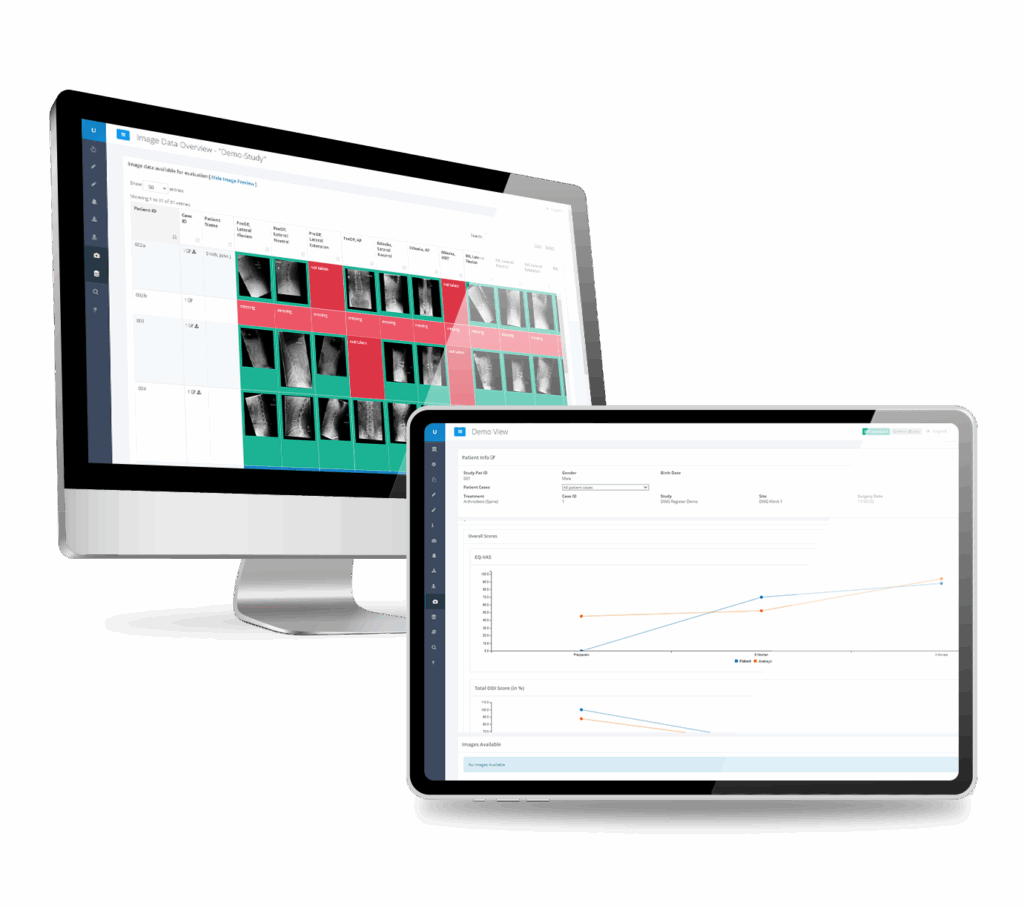
The RAYLYTIC Platform is a unified clinical trial platform that integrates ePRO, eCRF, and advanced imaging data to enforce protocol compliance and maximize data integrity. By reducing queries and operational costs, it accelerates regulatory approval timelines while generating robust, high-quality datasets that underpin your scientific legacy.
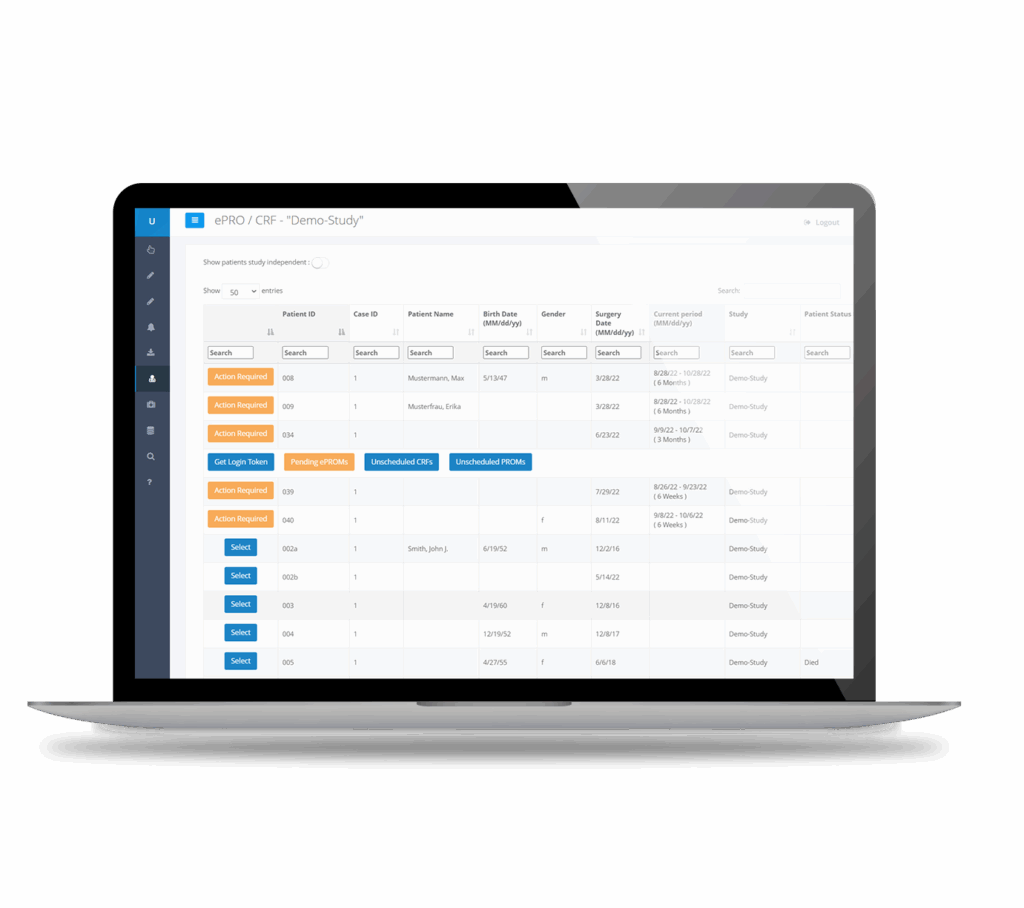
Our electronic data capture (EDC) system simplifies the collection of essential data for clinical trials, PMCF, and post-market surveillance.
- ePROs from diverse medical specialties
- Readily definable eCRFs and AE Forms
- Automated notes to patients and clinical sites
Our clinical trial management system (CTMS) automates workflows and error-prone tasks to maximize time and cost savings while mitigating risks to data integrity.
- Real-time status of data sets and study results
- Synched data between EDC and CTMS
- Drag-and-drop study designer
Imaging core lab services
We offer industry-leading imaging core lab services and are ready to support your trial end-to-end.
- Highly precise and accurate software- and AI-powered analysis of quantitative radiographic parameters
- Network of experienced radiologists for analysis of qualitative parameters
- Consultation for study design, endpoint selection, and data interpretation
Browser-based de-identification makes uploading images secure and straightforward.
- Integrated DICOM viewer
- Automatic image classification via AI-based algorithms
- Browser-based quality control
Looking to streamline your clinical trial?
Our platform for clinical trial management automates data capture and AI-supported analysis of medical data in one system.
Streamline your medical device trial management
Our integrated platform unifies EDC, PROMs, Image Management, and AI-driven Image Analysis to deliver comprehensive, high-fidelity datasets critical for medical device trials.
Our integrated EDC and PROMs software enables seamless collection and management of clinical data alongside patient-reported outcomes within a single, secure platform.
Automatically measures key radiographic metrics like Range of Motion, Implant Wear, and Device Migration—saving time and improving accuracy in your imaging assessments.
Gain 360-degree insights with regularly generated customizable reports highlight data status, enrollment and retention figures, and adverse events.
Collect real-world-data directly from patients and physicians. Continuously demonstrate the safety and efficacy of your device, inform treatment plans, and expand your commitment to evidence-based medicine.
Ensure protocol compliance and data integrity in medical device studies
The platform ensures strict protocol compliance and maintains data integrity throughout medical device studies by automating quality checks and standardizing data workflows.
Browser-based image data transfer, including GDPR-compliant, automated anonymization before transmission and storage
Interface between your system landscape (HIS, LIS, RIS, PACS) and UNITY software based on HL7 v2, HL7 v3, CDA and FHIR.
FDA CFR Part 11, GCP, Management system certified according to ISO 27001 and 13485


Ready to see how it works?
Discover how our partners are already using the RAYLYTIC Platform to conduct IDE studies, obtain market approval, and set up multicenter medical registries.
Simplify your clinical trial management.
For medical device manufacturers, valid results from clinical trials are essential for market approval. But these are often lengthy and very cost-intensive. The RAYLYTIC Platform offers numerous advantages that simplify clinical trial management and save resources.
Quick onboarding
Lower costs
World-class support
Save time
Intuitive operation
At RAYLYTIC we know that medical device manufacturers face a unique challenge: the long-term, efficient, and compliant collection of diverse data that demonstrate the objective safety and efficacy of their products.
This is why we developed the RAYLYTIC Platform– a scalable, modular platform for clinical trial management that automates the collection and analysis of ePROs, eCRFs, and medical imaging data. Together with our extensive repertoire of imaging core lab services, we ensure not only that your study runs smoothly – saving your trial up to six-figure amounts – but also generates the highest quality, scientific results for regulatory filings, post-market research, publications, and the evidence-based treatment of patients.