For clinical trial sponsors & CRos
Imaging Platform for Clinical Trials
The RAYLYTIC Platform is an advanced imaging platform for clinical trials that standardizes medical image management workflows and medical image analysis to reduce errors, shorten timelines, and generate regulatory-grade image-based evidence.
- Integrated imaging & EDC
- Audit-ready imaging workflows
- GDPR- and HIPAA-compliant
Trusted by top life science organizations





In clinical trials, imaging is often an afterthought.
But we know it's mission critical.
Too often, imaging data lives outside the core trial data model, leading to delays, inconsistencies, and unnecessary cost.
Common Challenges
Images exist outside the study database.
Save, transfer, and analyze imaging data within the full context of your study — organized by patient, timepoint, and site — so your team has complete visibility without leaving your clinical trial database.
High reconciliation effort between imaging and clinical data slows database lock.
Imaging is natively integrated into your EDC, eliminating reconciliation, reducing manual data management, and accelerating database lock. API connectivity with additional EDC systems coming soon.
Upstream image quality issues cause downstream re-work and queries.
At upload, images are assessed validated for correct timepoint, image type, and duplicates. Sites receive feedback, preventing costly re-scans and query cycles.
Inconsistent image analysis criteria across sites weaken your evidence.
Standardized reading protocols, and radiological endpoint definitions, and semi-automated measurement tools ensure consistent analysis across all sites and readers, strengthening regulatory submissions.
Long core lab read turnaround times delay enrollment decisions and milestones.
AI-assisted reading and intelligent workflow management reduce turnaround times by 50% or more, enabling faster enrollment decisions and keeping your trial on schedule.
Data silos prevent use of imaging insights across programs and post-market.
Our centralized imaging repository enables cross-trial analytics and post-market surveillance, turning imaging data into a strategic asset that compounds in value over time.
Imaging data, inside the study.
From upload to analysis, every image linked to the right patient, visit, and site within your clinical database.
Build & manage fully secure, scalable, and auditable imaging workflows.
The RAYLYTIC Platform is designed to support modern GCP expectations for imaging-intensive trials. Imaging data, workflows, and derived endpoints are structured to remain inspection-ready throughout the trial lifecycle.
- Smart image assessment to enhance compliance with imaging protocols
- Automatic, HIPAA-compliant image de-identification
- Virtually unlimited storage space for image databases
- Imaging eCRFs and image viewing in a single system
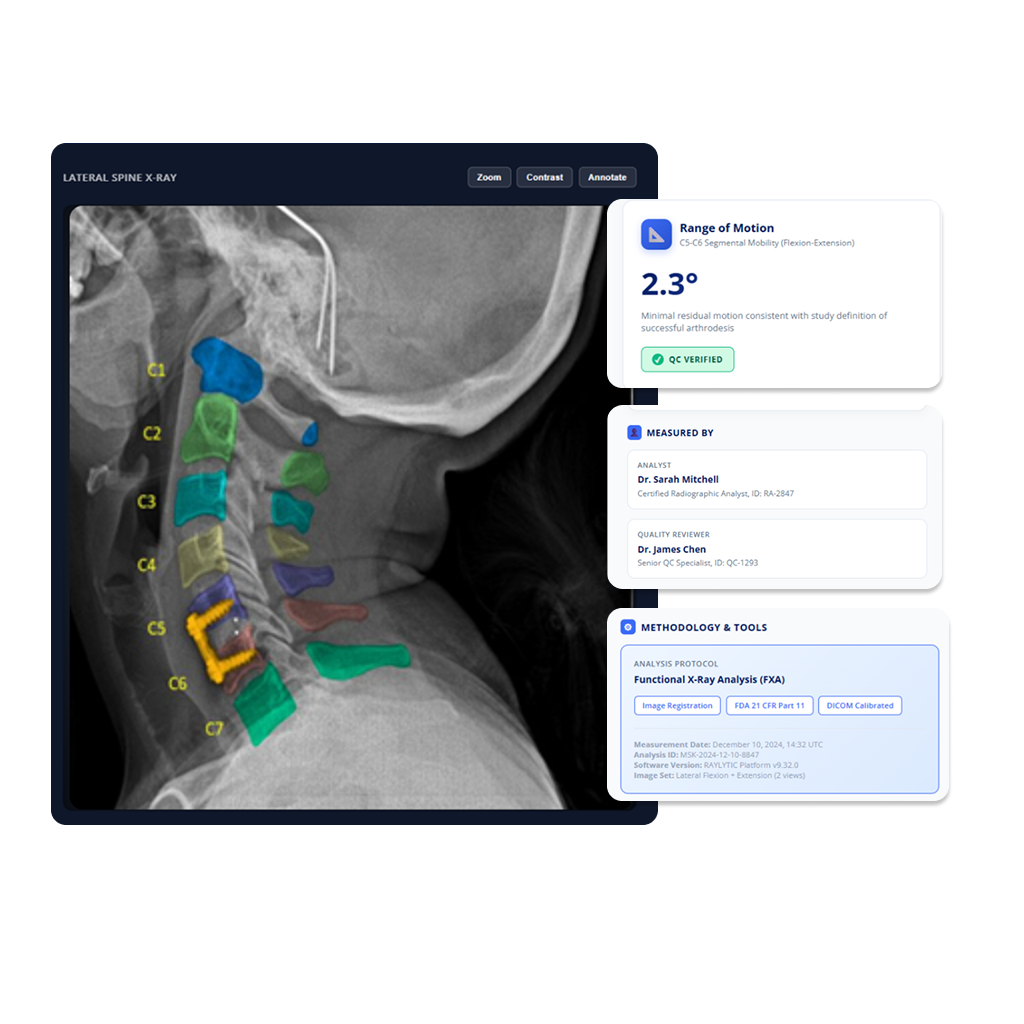
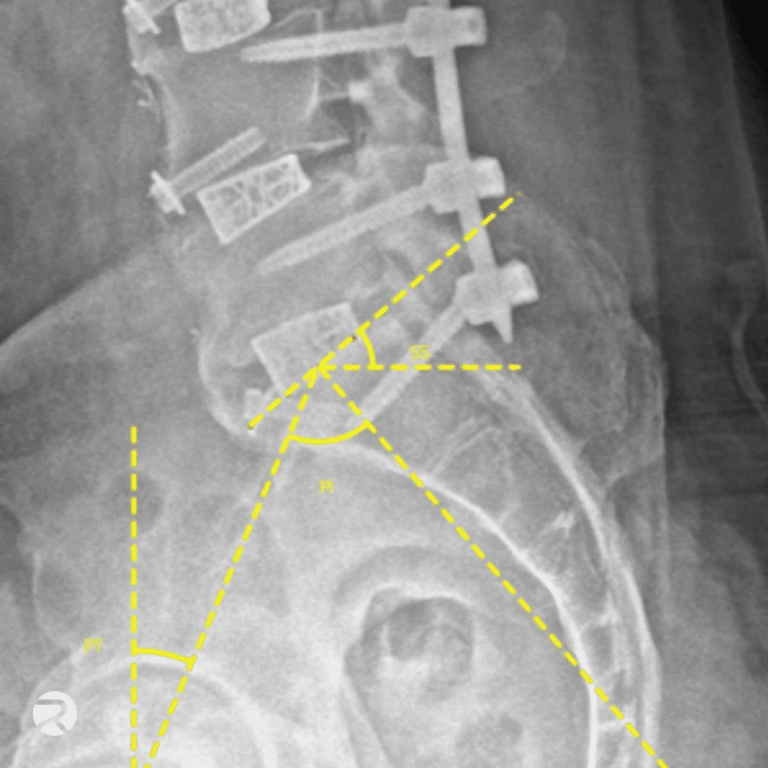
From subjective reads to standardized, objective measurements.
Traditional imaging approaches rely on manual reads and subjective scoring, introducing variability, delays, and uncertainty into imaging-driven endpoints. We enable you to generate objective, quantitative imaging evidence using AI-based image analysis.
- Assistance with endpoint definition and standardization for your unique study requirements
- AI-based image analysis for quantitative endpoint analysis
- Consistency and plausibility checks
Clinical Trials and Research
This sponsor achived 3× faster image analysis results with cost savings using RAYLYTIC.
The Complete RAYLYTIC Platform for Clinical Trial Sponsors
Imaging endpoints rarely stand alone. They must be interpreted alongside patient-reported and clinical outcomes, yet these data are often captured in separate systems and reconciled late. The RAYLYTIC Platform brings imaging, clinical data capture, and patient-reported outcomes together in a single, structured evidence model.
AI-Based Medical Imaging Management & Analysis
Integrated EDC & ePRO/eCOA
Security & Compliance
Accelerate clinical trials with AI-powered imaging analysis
- Deep learning algorithms for automated image analysis
- Automated measurements and quantification
- Multi-reader adjudication workflows
- DICOM viewer and integrated image management system
- Simultaneous image viewing with integrated image eCRF
- Standardized analysis protocols across sites
- Interactive dashboards & reports
Build, validate, and manage clinical forms with ease
- Intuitive form builder for rapid study setup
- Real-time validation rules and edit checks
- Complete audit trail and version control
- Multi-language support for global trials
- Role-based access control and user management
- Query management and resolution workflows
Enterprise-grade security built for healthcare
- 21 CFR Part 11 validated and certified
- GDPR and HIPAA compliant infrastructure
- SOC 2 Type II certified operations
- Role-based access control (RBAC)
- Complete audit trails for all user actions
What Makes Our Clinical Trial Imaging Solutions Different
50% faster review times without the bureaucracy of large CROs. Agile workflows that adapt to your study needs
Enterprise-grade quality without enterprise pricing: Significant savings through lean operations and smart automation
Direct access to senior experts, not ticket queues. Responsive support from people who know your study inside out
Eliminate variability with standardized, auditable measurements: regulatory-ready evidence backed by complete documentation